Services
Next generation technology for virus/viral vector imaging applied in antiviral screening, gene delivery, oncolytic virotherapies and the measurement of disinfection processes
Virology
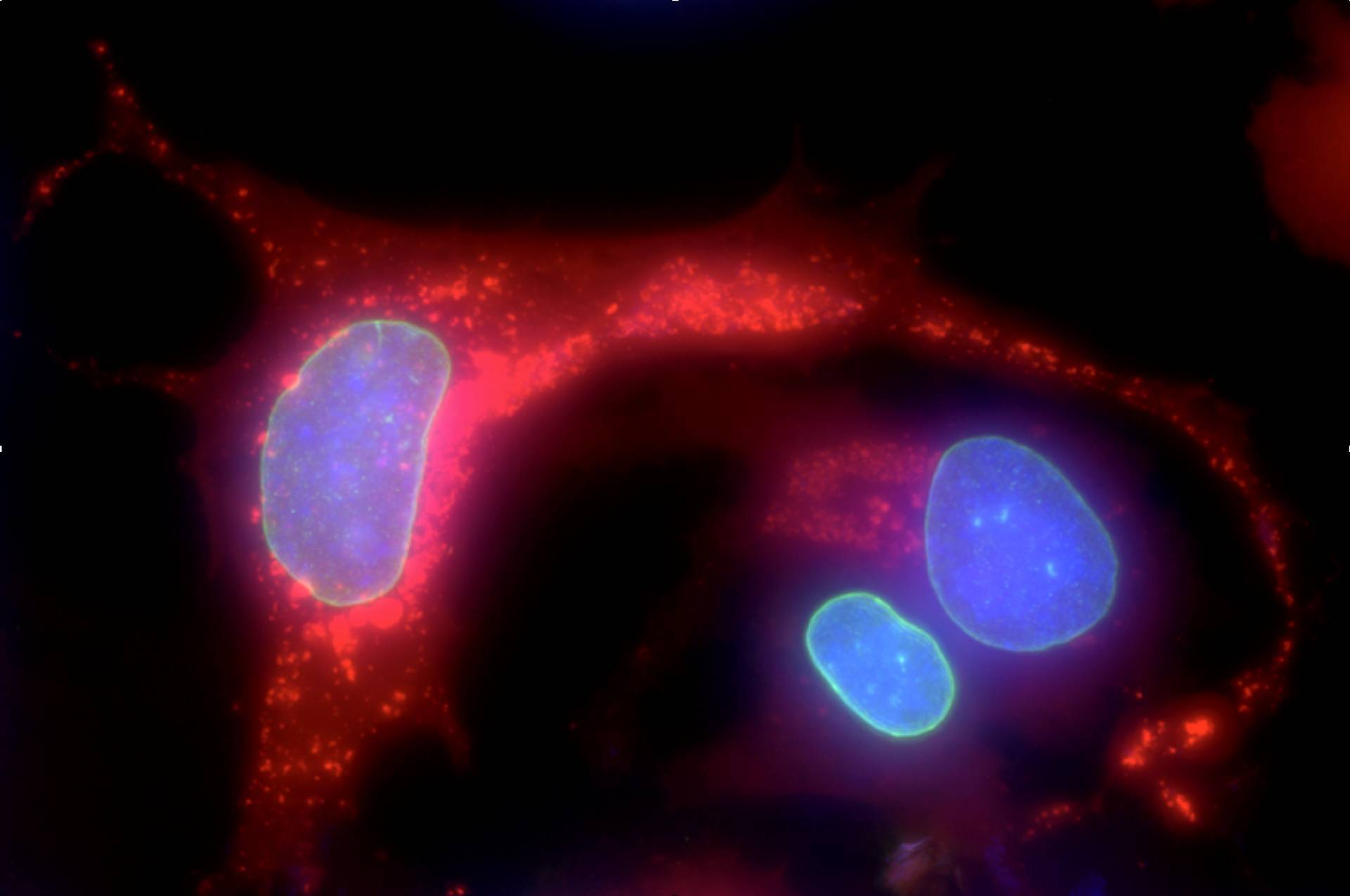
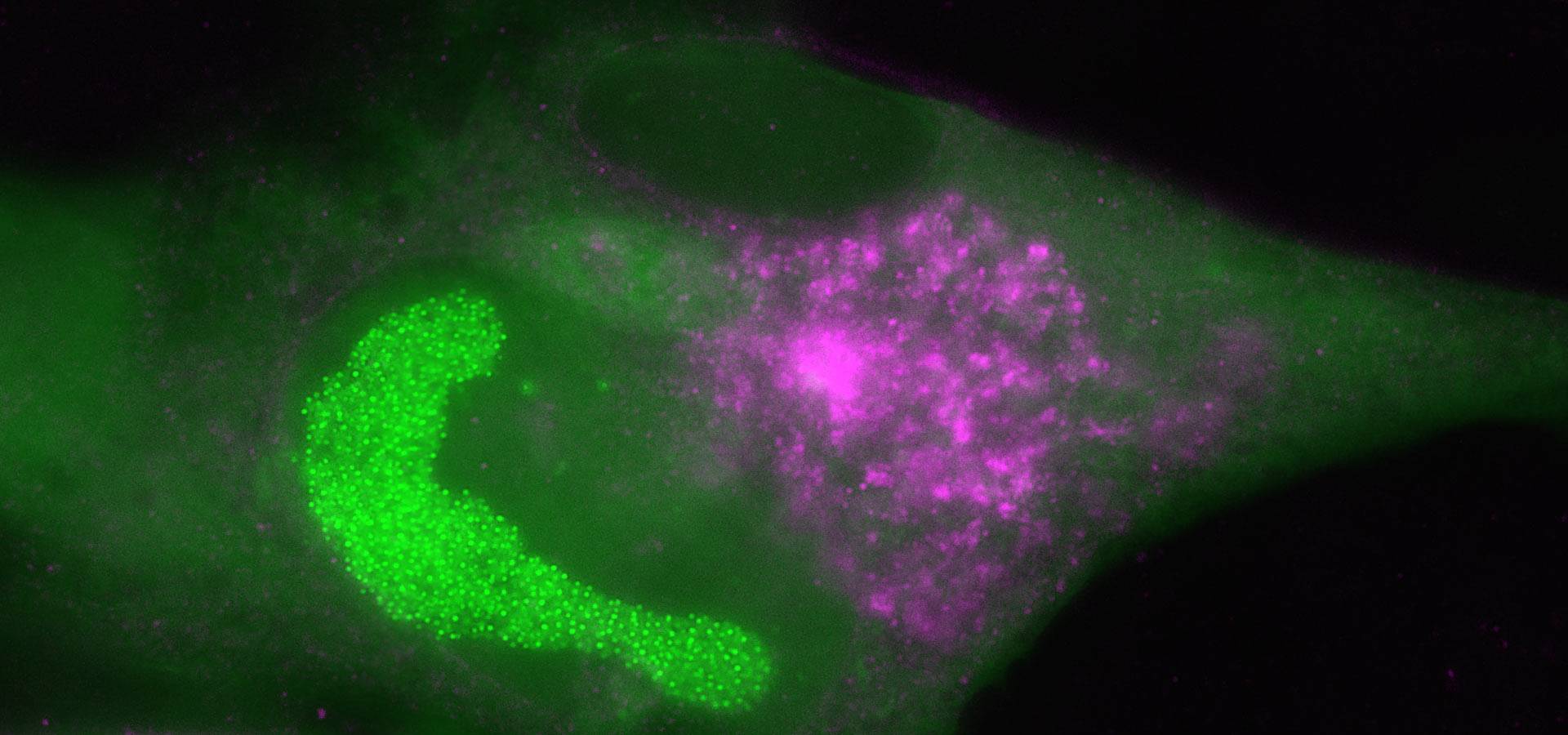
NeoVirTech has developed a breakthrough technology to directly visualize and quantify in real time virus/viral vector infection and replication in living cells. Application in antiviral discovery and disinfection procedures
Oncolytic virotherapies
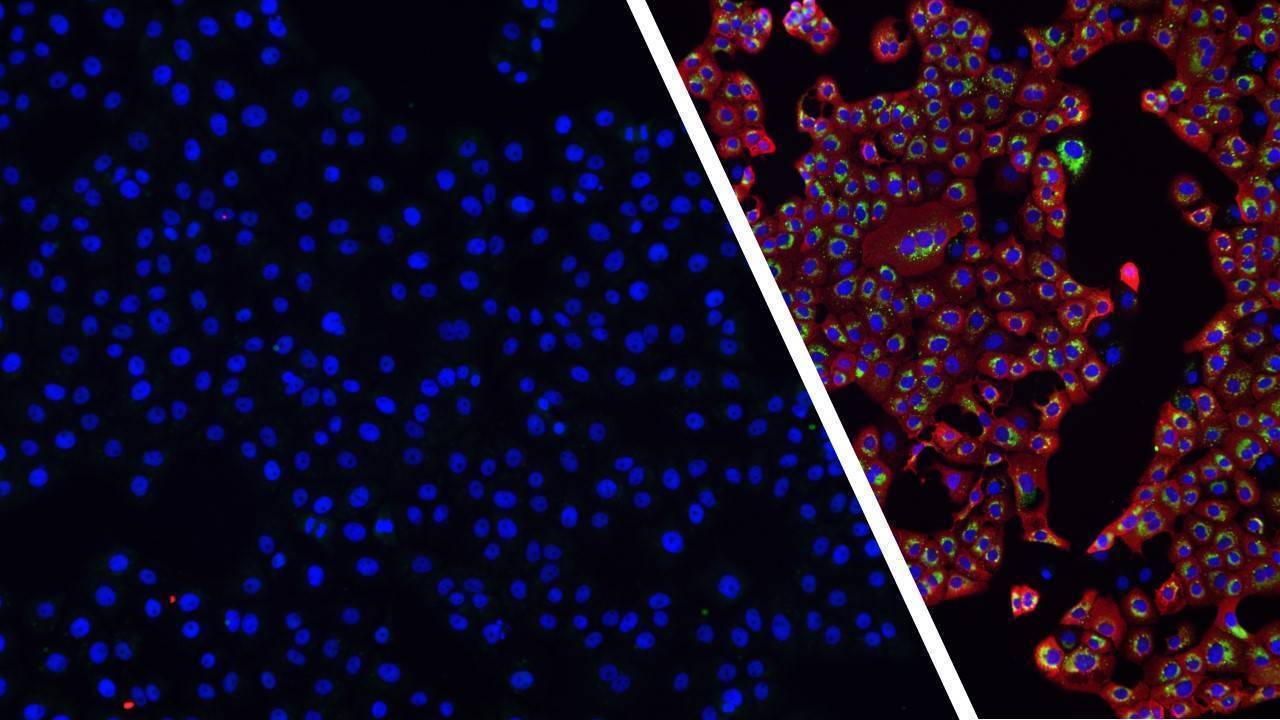
Conventional cancer treatments have limited effectiveness in many cases. Oncolytic therapy, based on the use of armed viruses directed against tumor cells, offers new perspectives in the fight against this global scourge.
Drug discovery
Using autofluorescent viruses, we can measure the impact of the presence of a molecule of interest on virus infection and replication capacities.
Disinfection testing
Our technology allows us to quickly calibrate and validate the development of disinfection devices
Imaging
We can offer different types of imaging and also image analysis whether in a viral or non-viral context, from high content imaging to high resolution imaging and light sheet microscopy.
News
Assets









A word from Franck, our CEO
Having the ability to directly visualize virus infection and replication in living cells using a breakthrough technology with minimum hands-on investment. This is what we propose at NeoVirTech. Our state of the art imaging and screening platform provides a rapid and powerful pipeline to discover compounds that will impact infection capacities and measure disinfection procedures on a large collection of viruses in the human and veterinary market. As our technology is image-based, the beauty and quality of the data generated is a tremendous asset to boost your research and expand your communication processes.